134x Filetype PDF File size 0.20 MB Source: sharadkvs11.files.wordpress.com
SCIENTIFIC LITERACY
THEME : PERIODIC CLASSIFICATION OF ELEMENTS
UNIT 1 : IUPAC NOMENCLATURE OF ELEMENTS
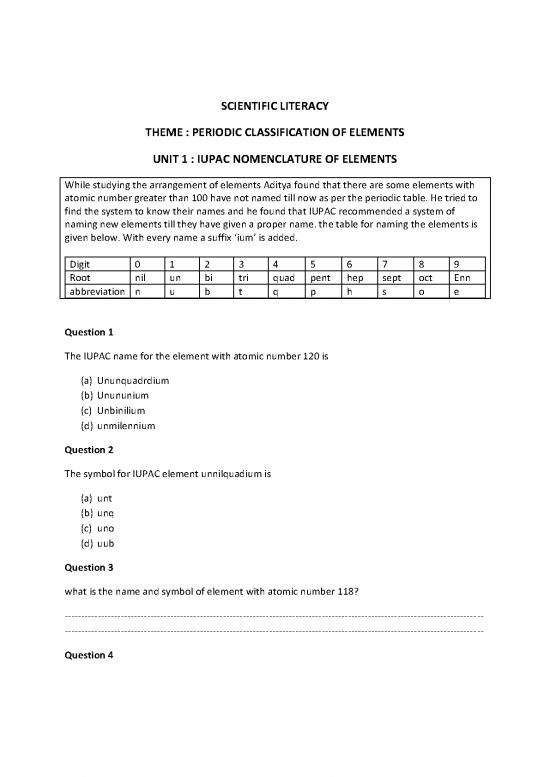
While studying the arrangement of elements Aditya found that there are some elements with
atomic number greater than 100 have not named till now as per the periodic table. He tried to
find the system to know their names and he found that IUPAC recommended a system of
naming new elements till they have given a proper name. the table for naming the elements is
given below. With every name a suffix ‘ium’ is added.
Digit 0 1 2 3 4 5 6 7 8 9
Root nil un bi tri quad pent hep sept oct Enn
abbreviation n u b t q p h s o e
Question 1
The IUPAC name for the element with atomic number 120 is
(a) Ununquadrdium
(b) Unununium
(c) Unbinilium
(d) unmilennium
Question 2
The symbol for IUPAC element unnilquadium is
(a) unt
(b) unq
(c) uno
(d) uub
Question 3
what is the name and symbol of element with atomic number 118?
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
Question 4
Write the name and atomic number of element with symbol uuu
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
UNIT 2 : ELEMENT POSITION IN PERIODIC TABLE
Three elements X, Y, and Z have atomic numbers 10, 21 and 32 respectively. Garima wants to
explain the properties of these elements according to the Modern Periodic Table. On studying
the modern periodic table, Garima could answer the following questions.
Question 1
in which group does the element X exist?
-------------------------------------------------------------------------------------------------------------------------------
Question 2
In which period does the element Y exist?
-------------------------------------------------------------------------------------------------------------------------------
Question 3
What is the valency of element Z?
-------------------------------------------------------------------------------------------------------------------------------
Question 4
Arrange the three elements X, Y and Z from non-metal to metal.
-----------------------------------------------------------------------------------------------------------------------------
UNIT 3 : CHEMICAL NATURE OF ELEMENTS ACCORDING TO POSITION IN
PERIODIC TABLE
Abdul and Raghav were discussing about the chemical nature of elements as they are given the
position in modern periodic table. Raghav wanted to know about the metallic and non-metallic
nature of the elements while Abdul was interested in oxidation of elements. After studying the
periodic table thoroughly and discussion they are able to understand the position of chemicals
and their chemical nature.
Question 1
Abdul said Ca20 will form oxide by reacting with oxygen as CaO2. Do you agree with Abdul?
Justify your answer.
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
Question 2
Raghav said that along the period of periodic table, metallic nature of elements decreases. Is he
correct?
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
Question 3
Iron forms its oxide as Fe O and Fe O Give reason for such behavior of iron.
2 3 3 4.
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
Question 4
Raghav said that between sodium hydroxide and potassium hydroxide, NaOH is more basic in
nature. According to you which is more basic NaOH or KOH?
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
UNIT 4 ANOMALY IN MODERN PERIODIC TABLE
Meenal studied the modern periodic table and found some anomalies in it. She found two
positions of hydrogen in periodic table while helium get a special position. Similarly after
lanthanum with atomic number 57, the next element is not Cesium with atomic number 58. But
there is another element Hafnium with atomic number 72. Similarly after Actinium with atomic
number 89, next element is not thorium with atomic number 90 but there is another element
Rutherfordium with atomic number 104.
She found that at the bottom of periodic table there are two series of elements in which these
missing elements are placed. She wanted to know the reason behind it.
Question 1
Hydrogen has given two positions in the periodic table. Give reason.
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
Question 2
Helium has not got a proper position in periodic table. It has only two electrons in its orbit but it
th nd
lies in 18 group. According to preparation of periodic table it should be in 2 group. Justify the
reason.
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
-------------------------------------------------------------------------------------------------------------------------------
Question 3
In 6th period, after lanthanum of atomic number 57, the next element is Hafnium with atomic
number 72 instead of Cesium of atomic number 58. Why?
no reviews yet
Please Login to review.