173x Filetype XLSX File size 0.14 MB Source: www.agriculture.gov.au
Sheet 1: Cover Sheet
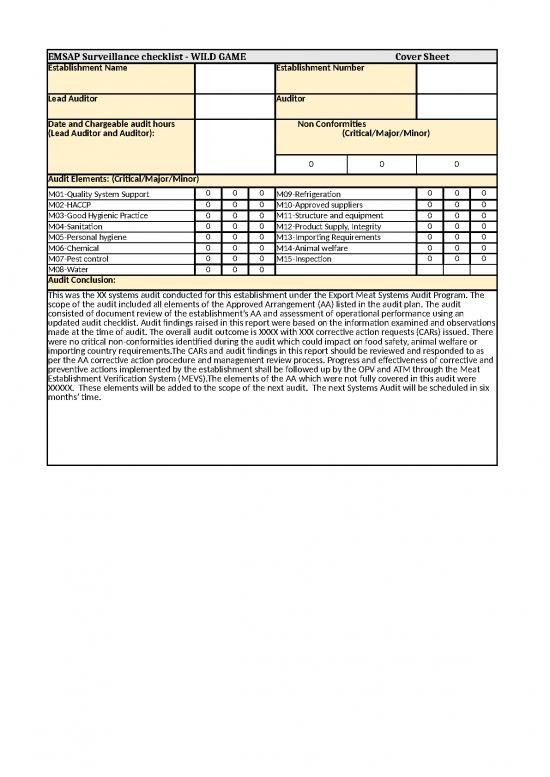
| EMSAP Surveillance checklist - WILD GAME | Cover Sheet | |||||||
| Establishment Name | Establishment Number | |||||||
| Lead Auditor | Auditor | |||||||
| Date and Chargeable audit hours (Lead Auditor and Auditor): | Non Conformities (Critical/Major/Minor) | |||||||
| 0 | 0 | 0 | ||||||
| Audit Elements: (Critical/Major/Minor) | ||||||||
| M01-Quality System Support | 0 | 0 | 0 | M09-Refrigeration | 0 | 0 | 0 | |
| M02-HACCP | 0 | 0 | 0 | M10-Approved suppliers | 0 | 0 | 0 | |
| M03-Good Hygienic Practice | 0 | 0 | 0 | M11-Structure and equipment | 0 | 0 | 0 | |
| M04-Sanitation | 0 | 0 | 0 | M12-Product Supply, Integrity | 0 | 0 | 0 | |
| M05-Personal hygiene | 0 | 0 | 0 | M13-Importing Requirements | 0 | 0 | 0 | |
| M06-Chemical | 0 | 0 | 0 | M14-Animal welfare | 0 | 0 | 0 | |
| M07-Pest control | 0 | 0 | 0 | M15-Inspection | 0 | 0 | 0 | |
| M08-Water | 0 | 0 | 0 | |||||
| Audit Conclusion: | ||||||||
| This was the XX systems audit conducted for this establishment under the Export Meat Systems Audit Program. The scope of the audit included all elements of the Approved Arrangement (AA) listed in the audit plan. The audit consisted of document review of the establishment’s AA and assessment of operational performance using an updated audit checklist. Audit findings raised in this report were based on the information examined and observations made at the time of audit. The overall audit outcome is XXXX with XXX corrective action requests (CARs) issued. There were no critical non-conformities identified during the audit which could impact on food safety, animal welfare or importing country requirements.The CARs and audit findings in this report should be reviewed and responded to as per the AA corrective action procedure and management review process. Progress and effectiveness of corrective and preventive actions implemented by the establishment shall be followed up by the OPV and ATM through the Meat Establishment Verification System (MEVS).The elements of the AA which were not fully covered in this audit were XXXXX. These elements will be added to the scope of the next audit. The next Systems Audit will be scheduled in six months’ time. | ||||||||
| NOTE: This section will automaticly take findings from rest of workbook | Audit Findings | ||||||||||||||||
| M01- Quality System Support | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M02- HACCP / Non conforming product | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M03- Good Hygienic Practice | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M04- Sanitation | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M05- Personal hygiene | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M06- Chemicals | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M07- Pest control | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M08- Water | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M09- Refrigeration | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M10- Approved suppliers | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M11- Structure and equipment | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M12- Product Supply Chain Integrity | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M13- Importing country requirements | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M14- Animal welfare and handling | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| M15- Inspection | |||||||||||||||||
| Audit Findings: | |||||||||||||||||
| EMSAP Surveillance checklist | M01 Quality System Support | ||||||||||||||||||||||
| Audit Evidence: Quality Management: Management review : Document control : Internal audit and Corrective Action : Training : | |||||||||||||||||||||||
| Audit Findings: | |||||||||||||||||||||||
| Audit Activity: | Select Rating | ||||||||||||||||||||||
| Quality Management: | |||||||||||||||||||||||
| Show me the establishment quality policy statement or commitment to compliance with the approved arrangement. How does this cover GHP, HACCP, product integrity, traceability, legislation and importing country requirements | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 1 | |||||||||||||||||||||||
| If the establishment uses AAOs, does the commitment include suitable wording eg AAOs perform inspection in accordance with department requirements | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 2(b) | |||||||||||||||||||||||
| Can you tell me about this establishment, show me the prepared plant profile which you would use as part of any foreign official visits | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 2 | |||||||||||||||||||||||
| Does the organisational chart and duty statements clearly describe responsibilities and back up arrangements. Who is responsible for any product recall | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 2 | |||||||||||||||||||||||
| Management Review: | |||||||||||||||||||||||
| Explain to me the management review process | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 5 | |||||||||||||||||||||||
| Review management review meeting records of at least the last two meetings, were outcomes clearly recorded, did the meeting agenda follow the approved SOP? was senior management present, are CCP CL failures and trends looked at (MHA, PHI) | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 5 | |||||||||||||||||||||||
| Does the management review process confirm the approved arrangement is working effectively and that the HACCP plan is current | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 5 | |||||||||||||||||||||||
| Document Control: | |||||||||||||||||||||||
| What is the process for amending the approved arrangement | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os 10.07, Schedule 1, 13,14,15 | |||||||||||||||||||||||
| Who is authorised to make changes | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os 10.07, Schedule 1, 13,14,15 | |||||||||||||||||||||||
| How are changes or new procedures implemented and verified. Does the AA update procedure include review of meat notices, market access advices and changes to MICoR | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os 10.07, Schedule 1, 13,14,15 | |||||||||||||||||||||||
| Show me the amendment register | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os 10.07, Schedule 1, 13,14,15 | |||||||||||||||||||||||
| Examine at least 5 amendments to verify the procedures in the document control SOP have been followed. | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os 10.07, Schedule 1, 13,14,15 | |||||||||||||||||||||||
| No variations to the approved arrangement that could affect food safety or product integrity have been implemented without prior approval from the department | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os 10.07, Schedule 1, 15 | |||||||||||||||||||||||
| How are records produced by the quality system stored for minimum 2 years | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 7 | |||||||||||||||||||||||
| Internal Audit and Corrective Action: | |||||||||||||||||||||||
| How is the annual internal audit schedule established | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 5 | |||||||||||||||||||||||
| Select a number of completed audits and examine the reports | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 5 | |||||||||||||||||||||||
| Is the internal auditor trained and not routinely part of the activity being audited | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 5 | |||||||||||||||||||||||
| Examine the list of corrective action records or other response to internal audit report findings. How is the corrective / preventive action process described in the QA manual applied and verified to ensure sustained compliance. Does investigation of cause link to planned response | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 4 & 5 | |||||||||||||||||||||||
| Are any non-conformity responses currently outstanding; if so are interim controls in place pending long term resolution | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 4 | |||||||||||||||||||||||
| Training: | |||||||||||||||||||||||
| Explain to me the training procedures from new staff induction to competence in specific work instructions | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 2 | |||||||||||||||||||||||
| How are existing staff kept current against the approved arrangement procedures (eg regular reinforcement training) or provided further training when procedures are amended or noncompliance is identified | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 2 | |||||||||||||||||||||||
| What records are available to supervisors to ensure only trained staff are used for each task during processing | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 2 | |||||||||||||||||||||||
| During establishment observations record names of a number of staff performing specific tasks and then verify evidence of suitable training | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 2 | |||||||||||||||||||||||
| Sight examples of training for key positions : MHA product and process monitoring, CCP CL monitoring, ESAM carcase swabbing, (where present AAOs,) pre-trim | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 2 | |||||||||||||||||||||||
| Does the approved training SOP reflect the procedures explained and records provided | Not Recorded | ||||||||||||||||||||||
| EC(WGM&MP)Os Schedule 2, 2 | |||||||||||||||||||||||
no reviews yet
Please Login to review.