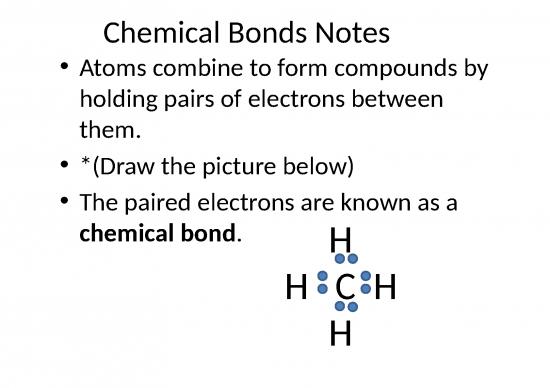
300x Filetype PPTX File size 0.71 MB Source: www.fsusd.org
Chemical Bond Classification
• Chemical bonds are classified by the way
the electrons are held between the
atoms that are bonding to each other.
Chemical Bond Classification
• Electrons can be held in a bond by
one of two ways:
–Electrons are equally shared by both
atoms. (Covalent)
–Electrons are “exchanged” from one
atom to another forming ions. (Ionic)
Properties of Covalent Bonds
• Covalent bonds occur between two
non-metal atoms.
• In covalent bonds electrons are shared
by the atoms involved in the bond.
Examples of Covalent Bonds
• Examples of compounds formed with
covalent bonds include:
–Hydrogen gas (H2)
–Ammonia (NH3)
–Large biological molecules such as
glucose (C H O ).
6 12 6
Properties of Ionic Bonds
• Ionic bonds occur between a metal
atom and a non-metal atom.
• In ionic bonds electrons are exchanged or
transferred from the metal atom to the
non-metal atom.
• After the transfer of electrons, both atoms
become oppositely charged, keeping both
atoms together (bonded).
no reviews yet
Please Login to review.